Synchron, which has developed an endovascular brain computer interface that can access every corner of the brain using its natural highways, the blood vessels has secured $75 million in a Series C financing round led by ARCH Venture Partners.
The Series C funding brings the total amount raised by the company since inception to $145 million.
The firm will use the fresh round of funding to further the development of its platform Synchron SwitchTM BCI, and launch a pivotal clinical trial. Ari Nowacek from ARCH Venture Partners will join Synchron’s Board, and ARCH Co-founder and Managing Director Robert Nelsen will join as a Board Observer.
“We have an opportunity to deliver a first-in-class commercial BCI.” the company stated.
The problem of paralysis is much larger than people realise. 100 million people worldwide have upper limb impairment,” said Tom Oxley, CEO & Founder, Synchron. “We are extremely excited to work with ARCH and this world-class syndicate to bring this technology to the people who need it.”
Synchron advances BCI Platform, readies to commercialize the product
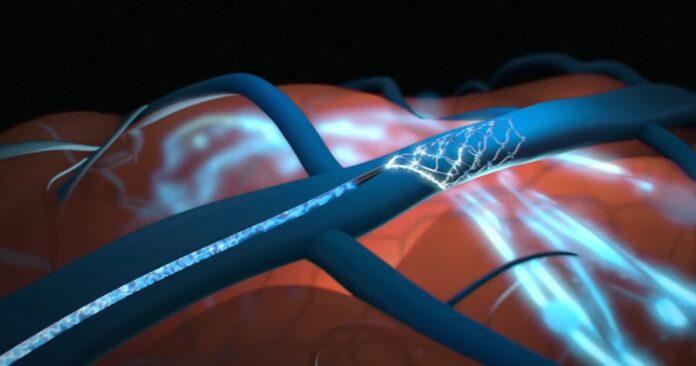
The US-based firm founded in 2012 has been developing a BCI platform that avoids the need for open brain surgery by using a minimally-invasive procedure. The Synchron SwitchTM brain computer interface is implanted in the blood vessel on the surface of the motor cortex of the brain via the jugular vein through a minimally-invasive endovascular procedure.
The Synchron Switch™ brain computer interface is implanted in the blood vessel on the surface of the motor cortex of the brain via the jugular vein, through a minimally-invasive endovascular procedure. Once implanted, it is designed to detect and wirelessly transmit motor intent out of the brain, restoring a capability for severely paralyzed patients to control personal devices with hands-free point-and-click.
The firm has an ongoing US clinical trial, COMMAND, that assesses the impact on daily tasks such as texting, emailing, online shopping, and telehealth services. The FDA granted Breakthrough Device designation to Synchron in August 2020 and an Investigational Device Exemption in July 2021. The first US patient was implanted in July 2022 at Mount Sinai in New York. View Synchron CEO 2022 TED Talk, “A brain implant that turns your thoughts into text.”
ARCH has also on-boarded new investors Gates Frontier, Reliance Digital Health Limited, Bezos Expeditions, Greenoaks, Alumni Ventures, Moore Strategic Ventures, and Project X.
Existing investors including Khosla Ventures, METIS, Forepont Capital Partners, NeuroTechnology Investors, ID8 Investments, Shanda Group, and the University of Melbourne participated in the round.